Air toxics

Air toxics or 'hazardous air pollutants' are gases, particles or aerosols present in the air at levels where they pose a hazard to human health, plant or animal life.
Air toxics include volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), heavy metals and aldehydes.
The most common air toxics are:
These substances are often among the highest air toxics on a mass emissions basis, and may have significant health impacts such as cancer or respiratory, reproductive and developmental effects.
Sources of air toxics
Air toxics can come from:
- combustion processes, such as engines, power stations, bushfires and hazard reduction burning
- non-combustion activities where solvents are used, such as in cleaning and paint manufacture industries.
Monitoring air toxics
The Queensland Government monitors benzene, toluene, xylene and formaldehyde levels in Gladstone, and previously in South East Queensland between 2000 and 2020. Motor vehicle and industrial emissions are the major sources of pollutants at these locations.
Benzene, toluene, xylenes and formaldehyde are measured using Differential Optical Absorption Spectroscopy (DOAS) instrumentation.
PAHs can be monitored when necessary. When measured, they are collected using a combination of a quartz filter and sorbent cartridge, then analysed for up to 19 different compounds using a technique known as Gas Chromatography/Mass Spectrometry (GC/MS). Gas chromatography separates the different PAHs and mass spectroscopy identifies each individual PAH.
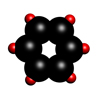
Benzene
Benzene is an organic compound with a molecular formula C6H6. In its normal state, it is a colourless liquid with a boiling point of 80.1°C.
It occurs naturally in fossil fuels and enters the atmosphere from natural processes and from human activities that involve the combustion of organic matter, such as wood, coal and petroleum products.
Vehicle emissions are the major source of benzene in the environment, although industries such as petroleum refining, and evaporation from fuel storage facilities and service stations are other ways benzene enters the atmosphere.
Tobacco smoke contains benzene and accounts for approximately 50% of a smoker's total exposure.
Health effects
Long-term exposure to benzene results in an increased incidence of blood and immune system disorders, including anaemia and leukaemia. At typical ambient concentrations, benzene does not have any short-term or acute health effects.
Air quality guidelines
The Environmental Protection (Air) Policy 2019 (EPP Air) objective for benzene is a maximum concentration of 0.002 parts per million (ppm) over a 1-year period.
The National Environment Protection (Air Toxics) Measure sets a monitoring investigation level for benzene at an annual average of 0.003ppm.
The monitoring investigation level value is the level of benzene below which exposure for the given averaging time does not constitute a significant health risk. However, short-term excess exposure does not mean adverse health effects automatically occur.
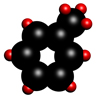
Toluene
Toluene is also a colourless organic liquid with a boiling point of 110.6°C and a molecular formula C6H5.CH3. Burning organic matter, such as wood, coal and petroleum products generates toluene, and it occurs naturally in crude oil.
Motor vehicle emissions are the main source of toluene in the urban air environment, although evaporative losses from fuel storage facilities and service stations, as well as the use of toluene-based solvents and thinners are other contributors.
Like benzene, toluene is a component of tobacco smoke. The highest concentrations of toluene usually occur indoors from the use of household products containing toluene, such as paints, thinners, adhesives and cigarette smoke.
Health effects
The main health effect associated with toluene exposure is damage to the central nervous system.
There is currently no evidence that toluene causes cancer.
Toluene may affect reproduction, foetal development and hormone balance when exposed to concentrations significantly higher than typical outdoor levels, either through occupational exposure or through solvent abuse.
These effects do not occur at toluene levels typically experienced in ambient air.
Air quality guidelines
The Environmental Protection (Air) Policy 2019 (EPP Air) objectives for toluene are a maximum concentration of 1ppm over a 24-hour period and a maximum concentration of 0.1ppm over a 1-year period.
The National Environment Protection (Air Toxics) Measure sets a monitoring investigation level for toluene at a 24-hour average of 1ppm and an annual average of 0.1ppm.
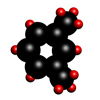
Xylene
Xylene is a colourless organic liquid with a boiling point of 138.5°C and a molecular formula CH3.C6H4.CH3.
It exists in three forms:
- ortho-xylene
- meta-xylene
- para-xylene.
Burning organic matter, such as wood, coal and petroleum products generates xylene, and it also occurs naturally in crude oil. The composition of xylene produced from petroleum is a mixture containing approximately 40% m-xylene, 20% o-xylene, 20% p-xylene, and 20% ethyl benzene.
Motor vehicle emissions are the predominant source of xylene in the urban air environment.
Evaporation from petroleum fuels storage facilities and service stations, and the use of products containing xylene-based solvents and thinners, are other ways xylene enters the air environment.
Health effects
Exposure causes eye, nose and throat irritation as well as neurological effects such as slowed reaction, short-term memory loss and the loss of body coordination.
There is no evidence that xylene causes cancer.
The above effects do not occur at xylene levels typically experienced in ambient air.
Air quality guidelines
The Environmental Protection (Air) Policy 2019 (EPP Air) objectives for xylene are a maximum concentration of 0.25ppm over a 24-hour period and a maximum concentration of 0.2ppm over a 1-year period.
The National Environment Protection (Air Toxics) Measure sets a monitoring investigation level for xylene at a 24-hour average of 0.25ppm and an annual average of 0.2ppm.
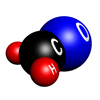
Formaldehyde
Formaldehyde in its normal state is a colourless gas with a molecular formula of CH2O. It is more commonly known as formalin, an aqueous solution used to disinfect and preserve tissue and specimens.
Low levels of formaldehyde are part of naturally occurring decomposition processes.
In urban environments formaldehyde emission sources include:
- motor vehicle exhaust
- domestic solid fuel and gas combustion
- goods manufactured with formaldehyde-based glues and resins
- tobacco smoke.
The last two sources are important indoor air quality issues.
Photochemical reactions involving oxidation of hydrocarbon compounds can also produce formaldehyde.
Health effects
Exposure to moderate levels of formaldehyde (1-3ppm) can result in eye, nose and upper respiratory tract irritation.
Formaldehyde has an annoying odour at lower concentrations.
Although classified as a probable human carcinogen, typical ambient concentrations of formaldehyde are unlikely to cause cancer.
Air quality guidelines
The Environmental Protection (Air) Policy 2019 (EPP Air) objective for formaldehyde is a maximum concentration of 0.04ppm averaged over a 24-hour period.
The National Environment Protection (Air Toxics) Measure sets a monitoring investigation level for formaldehyde at a 24-hour average of 0.04ppm.

Polycyclic aromatic hydrocarbons (PAHs)
Polycyclic aromatic hydrocarbons form during the incomplete burning of organic material. Most PAHs have no known use other than in some medicines and in industrial processes.
Benzo(a)pyrene is the best studied and one of the most toxic of all PAHs. It is often used as an indicator, or marker, for this group of pollutants as there is little information on human exposure to any single, pure PAH.
Health effects
Exposure to PAHs may irritate the eyes, skin and respiratory tract and at high levels may cause headaches, nausea, vomiting and abdominal pain.
A number of PAHs are likely to cause cancer in humans. Benzo(a)pyrene is associated with lung cancer, with the risk increasing in the presence of other substances, such as in the case of tobacco smoke.
Air quality guidelines
The Environmental Protection (Air) Policy 2019 (EPP Air) objective for benzo(a)pyrene (used as a marker for PAHs) is a maximum concentration of 0.3 nanograms per cubic metre (ng/m3) averaged over a 1-year period.
The National Environment Protection (Air Toxics) Measure sets a monitoring investigation level for benzo(a)pyrene at an annual average of 0.3ng/m3.


